1. Introduction
Disinfection is commonly divided into three levels of activity: high level disinfection (HLD), intermediate level disinfection (ILD), and low level disinfection (LLD). High level disinfection is defined as the elimination or destruction of all forms of microbial life except high numbers of bacterial spores. Intermediate level disinfectants exhibit a tuberculocidal effect together with inactivation of other vegetative bacteria, fungi, and most viruses. Lastly, a low level disinfectant lacks tuberculocidal activity, but most vegetative bacteria and some fungi and some viruses are inactivated. This classification scheme is summarized in Table 1. This post presents a quick discussion on glutaraldehyde and hydrogen peroxide, two chemicals widely used as intermediate and high level disinfectants in hospital settings. To make sure that the post remains steeped in practical terms rather than mere theoretical information, I chose to briefly review two applications of these disinfectants, namely, the use of glutaraldehyde in the decontamination of endoscopes and the application of hydrogen peroxide in the disinfection of contact lenses. Let’s begin with glutaraldehyde.
Table 1. Disinfection levels

2. Glutaraldehyde and Endoscopes
Glutaraldehyde (GA) is a simple molecule with two aldehyde groups. GA solutions are colorless, although many contain a dye to give an amber color, and have a characteristic strong “rotten apple” odor. Formulations are provided in concentrations ranging from 1.5 to 3.5%. GA solutions are sold under acidic conditions and subsequently ‘activated’ – that is, made alkaline with pH 8 – prior to use. GA is a reactive, cross-linking biocide whose stability and action are drastically affected by acidity. A substantial boost in antimicrobial activity is achieved as the solution pH rises from 4 to 9; this, however, is often accompanied by a concomitant reduction in shelf life, solutions at pH 8 and above losing activity within 4 weeks (McDonnell, 2007).
Glutaraldehyde has a broad spectrum of antimicrobial activity; it is fungicidal, virucidal, and bactericidal at < 10 min at 2%, with longer contact times required for sporicidal activity. Investigators have shown that a 2% or greater aqueous solution of GA, buffered to pH 7.5 to 8.5 with sodium bicarbonate, was effective in killing vegetative bacteria in less than 2 minutes; M. tuberculosis, fungi, and viruses in less than 10 minutes; and spores of Bacillus and Clostridium species in 3 hours (Scott and Gorman, 2001). In a comparative study on GA and other aldehydes, Power and Russell (1990) concluded that the sporicidal activity of glutaraldehyde is superior to those of formaldehyde and butyraldehyde. Sporicidal activity of GA is dependent on pH, alkaline solutions being more effective than acid ones. As the temperature increases, the difference in sporicidal activity between alkaline and acid solutions is reduced.
Reports of microbial resistance to glutaraldehyde include Mycobacterium chelonae and Mycobacterium avium, but more recent research has confirmed the mycobactericidal action of this compound (Moore and Payne, 2004). Another problem organism is Clostridium parvum; one paper has revealed that C. parvum cysts remain viable after being exposed to 2.5% GA for the time prescribed in endoscope disinfection guidelines (Wilson and Margolin, 1999).
Although endoscopes represent a valuable diagnostic and therapeutic tool in modern medicine and the incidence of infection associated with use has been reported as very low (about 1 in 1.8 million procedures), more healthcare-associated outbreaks have been linked to contaminated endoscopes than to any other medical device. Until the 1990s, high level disinfection of endoscopes was normally undertaken by manual or automatic scope cleaning plus a 10 to 20 minute soak in 2% alkaline glutaraldehyde. After the Environmental Protection Agency (EPA) carried out further tests in the 1980s, resistance of some microorganisms, especially Mycobacterium species, to conventional disinfection protocols was verified. Accordingly, the Food and Drug Administration (FDA) updated its regulations and established that only quantitative tuberculocidal testing methods and test results showing 100% kill of M. tuberculosis would be acceptable for high level disinfectant claims. The first product to receive clearance under the new rule was Cidex, a GA solution manufactured by Johnson and Johnson, in 1994. Shortly after, the manufacturer implemented a product label change, stating that to achieve a 100% kill of M. tuberculosis a 45-minute soak at 25oC was advised. Urayama et al. (1996) put the new recommendation to the test, but could not determine whether the extra soaking time in fact led to high-level disinfection effects.
In the UK, recommended exposure times to glutaraldehyde in endoscope disinfection are less stringent. In 1988, the British Society of Gastroenterology (BSG) Working Party published recommendations for cleaning and disinfection of equipment for gastrointestinal flexible endoscopy; aldehyde preparations (2% activated glutaraldehyde and related products) were recommended as first line microbicidal disinfectants and a four minute immersion or contact time was suggested for inactivation of vegetative bacteria and viruses (Cowan et al., 1998). In late 1994, the BSG Working Party reconvened and, in view of the fact that no transmission of infection had been reported under the existing guidelines, decided to maintain the rule established in the previous decade.
There is also some debate on the concentration of the glutaraldehyde solution used in endoscope disinfection. Most facilities use 1%, 2%, or 2.5% solutions; while alkaline 1% and alkaline 2% GA solutions were found to have similar virucidal effects in one study (Spire et al., 1984), another group of investigators revealed that 2% alkaline GA is more active than 1% alkaline GA in the decontamination of HIV from surfaces (Hanson et al., 1989). Yet another study suggested a 1% GA solution may not produce sufficiently adequate inactivation factors against some crucial microorganisms, including Pseudomonas aeruginosa (Jetté et al., 1995).
Glutaraldehyde fumes are eminently irritating and toxic to skin, mucous membranes and especially the respiratory tract. The most well-documented adverse effect of exposure to GA vapor is occupational asthma, a chronic condition characterized by bronchial hyperresponsiveness. Reactions can be either immediate or delayed, with a latent period ranging from a few weeks to several years after the onset of exposure. Heightened levels of occupational asthma have been verified in healthcare workers that frequently handle this compound (Di Stefano et al., 1999). Respiratory tract irritation is observed at concentrations as low as 0.3 ppm (McDonnell, 2007). Contact dermatitis was the most prevalent work-related symptom encountered in a sample of endoscopy nursing staff routinely exposed to GA (Vyas et al., 2000).
The American Conference of Governmental Industrial Hygienists (ACGIH) has adopted a ceiling threshold limit (TLV-C) for glutaraldehyde of 0.05 ppm. A TLV-C represents an airborne concentration that should not be exceeded during any part of the working shift. The National Institute for Occupational Safety and Health (NIOSH) has established a recommended exposure limit of 0.2 ppm as a ceiling limit for GA (NJDHSS, 1997). Measurements of healthcare exposure to glutaraldehyde vapor during high-level disinfection have been reported to range from zero to 0.2 ppm (OSHA, 2006). One study reported exposures of up to 0.15 ppm in routine endoscope disinfection (Waters et al., 2003).
Hands should be protected from contact with glutaraldehyde solutions. Nitrile and butyl rubber are the materials most impervious to GA (NJDHHS, 1997). Polyethylene and spun-bonded polypropylene coated with polyethylene provide adequate protection for several hours. Polyvinyl chloride and neoprene do not afford adequate protection against GA solutions (NJDHHS, 1997; NIOSH, 2001). Isolation gowns, lab coats, or aprons and sleeve covers, which are made of appropriate materials, should be used to provide additional protection. Splash-proof goggles and/or full face shields should be used whenever working with GA.
Toxicology studies carried out in the framework of the US National Toxicology Program found no evidence of carcinogenicity associated with exposure to glutaraldehyde in rats and mice (NTP, 1999). Likewise, Collins et al. (2006) found no increased rates of respiratory tract cancer or leukemia in a small sample of workers employed in a GA producing plant.
Spent glutaraldehyde is a hazardous waste, and care should be taken when disposing of this material so that the environment and human health are protected. If diluted to less than 5 ppm, the molecule can undergo natural decomposition. If dilution to this level is not practical, chemical deactivation may be employed. A 1 – 2% glycine-based solution can neutralize GA and convert it into pH-neutral, non-hazardous wastes (Dow Chemical, 2003); addition of sodium bisulfite at 2 – 3 parts (by weight) of NaHSO3 per part of active GA is also effective.
3. Hydrogen peroxide and contact lenses
Hydrogen peroxide (HP) is a strong oxidizing agent and is probably one of the most widely used biocides for medical, industrial, and household applications. It is commercially available as a colorless liquid at various dilutions, ranging from 3 to 90%, in water. Pure HP is relatively stable, but most dilutions contain a stabilizer (e.g., acetanilide or phenol) to prevent decomposition. HP has been employed in the food industry for surface disinfection and sterilization.
Depending on the concentration used, hydrogen peroxide is effective against bacteria, yeasts, viruses, and bacterial spores. Destruction of spores is greatly enhanced with both a rise in temperature and an increase in concentration. One manufacturer published data on its stabilized hydrogen peroxide solution, stating that at 20oC the product was fungicidal in 5 minutes, mycobactericidal in 20 minutes, and sporicidal in 6 hours (Oral Health, 2000). In a comparative study on the effect of eight germicides on the disinfection of Bacillus subtilis from different types of medical devices, HP produced superior results, achieving 99.9% inactivation after 30 minutes exposure at 20oC (Sagripanti and Bonifacino, 2006); glutaraldehyde came second. One of the most important biocidal characteristics of HP is that it has been shown to inactivate Cryptosporidium spores (Weir et al., 2002).
Since hydrogen peroxide can be neutralized by catalase and peroxidases, researchers have attempted to evaluate the effect of HP on organisms with high levels of activity for these enzymes. Schaeffer et al. (1980) found that organisms with high cellular catalase activity such as Staphylococcus aureus and Proteus mirabilis required 30 to 60 min of exposure to 0.6% HP to be reduced from 108 to 1 CFU/mL, while species with lower catalase activity such as E. coli and Pseudomonas sp. demanded only 15 min of exposure. More recently, Ríos-Castillo et al. (2017) showed that activity of HP against catalase positive bacteria such as S. aureus can be enhanced when the compound is formulated with small additions of benzalkonium chloride, ethyl alcohol, sodium benzoate, and other secondary agents.
Hydrogen peroxide has been shown to be effective against the opportunistic pathogen Acanthamoeba, the causative agent of Acanthamoeba keratitis. Acanthamoeba cysts were shown to withstand desiccation, temperature extremes ranging from -20 to 56oC, and exposure to 50 ppm free chlorine for 18 h (Chalmers, 2014). These protozoans cause a destructive ocular infection in previously healthy persons known as Acanthamoeba keratitis (AK). The incidence of this disease is greatest among contact lens wearers, which account for 95% of cases (Stehr-Green et al., 1989; Radford et al., 1995); use of non-sterile saline solutions and the rinsing of lenses in tap water are major predisposing factors. It is estimated that one in 30,000 soft contact lens users will develop AK (Seal, 2003).
One study found a disinfectant containing 3% hydrogen peroxide to be slightly more effective in killing some species of Acanthamoeba than other compounds, including commercial solutions containing thimerosal and chlorhexidine (Brandt et al. 1989). By contrast, an earlier investigation found that cysts of A. castelanii (but not those of A. polyphaga) were killed by a 4 h exposure to 0.005% chlorhexidine, whereas 3% HP was ineffective following exposure times of 10 and 30 min (Ludwig et al., 1986). Another comparative experiment found that HP is effective against Acanthamoeba trophozoites but less effective against 2-week-old cysts, thereby suggesting that the compound may not afford adequate protection against mature cysts (Kobayashi, 2011).
Contact lens solutions are available in ‘one-step’ and ‘two-step’ formulations; the former have been introduced to do away with the need to add a neutralizing tablet to the solution after cleaning the contact lens case. However, as it pertains to one-step solutions, experiments have produced worrisome results. In one study, Acanthamoeba cysts remained viable after an eight-hour soak in a one-step 3% hydrogen peroxide system, whereas a two-step 0.6% hydrogen peroxide system showed excellent amoebicidal effects (Hiti et al., 2002). A few years later, the same researchers published an enlarged study, investigating the biocidal activity of nine one-step 3% HP systems and 2 two-step HP systems (concentrations 0.6 and 3.0% H2O2); after an 8 h soaking period, both two-step systems eradicated the cysts of the Acanthamoeba strains tested, whereas the nine one-step systems showed poorer results (Hiti et al., 2005).
A toxicity analysis revealed that one hydrogen peroxide solution at concentration levels of disinfection interest may be ascribed to EPA toxicity category IV, which encompasses non-toxic, non-irritant substances (Omidbakhsh and Sattar, 2006). Although HP is generally not toxic, acute contact with high-concentration solutions may cause irritation. Acute inhalation of HP leads to irritation of the nose, throat and respiratory tract. In severe cases, bronchitis and pulmonary edema may follow. The International Association for the Research on Cancer (IARC) has found limited evidence in experimental animals for the carcinogenicity of HP. Overall, it is not classifiable as to its carcinogenicity to humans (group 3) (IARC, 1999).
The most important hazard of hydrogen peroxide is perhaps not its effect on humans, but on materials, as this compound is corrosive to a number of metals and alloys, including copper, zinc, and brass. HP solutions in concentrations greater than 8% are classified as corrosive liquids. Aluminum alloy equipment have been used in distilling, shipping, and storing HP; the American Society for Metals’ Handbook on Corrosion Data notes that aluminum alloy 1060 has been the choice for long-term storage, while the 5XXX series alloys have been selected for short-term storage (ASM International, 1995).
Hydrogen peroxide is not itself flammable but can cause spontaneous combustion of flammable materials and continued support of the combustion because it liberates oxygen as it decomposes. It is not considered to be an explosive; however, when mixed with organic chemicals, hazardous impact-sensitive compounds may result.
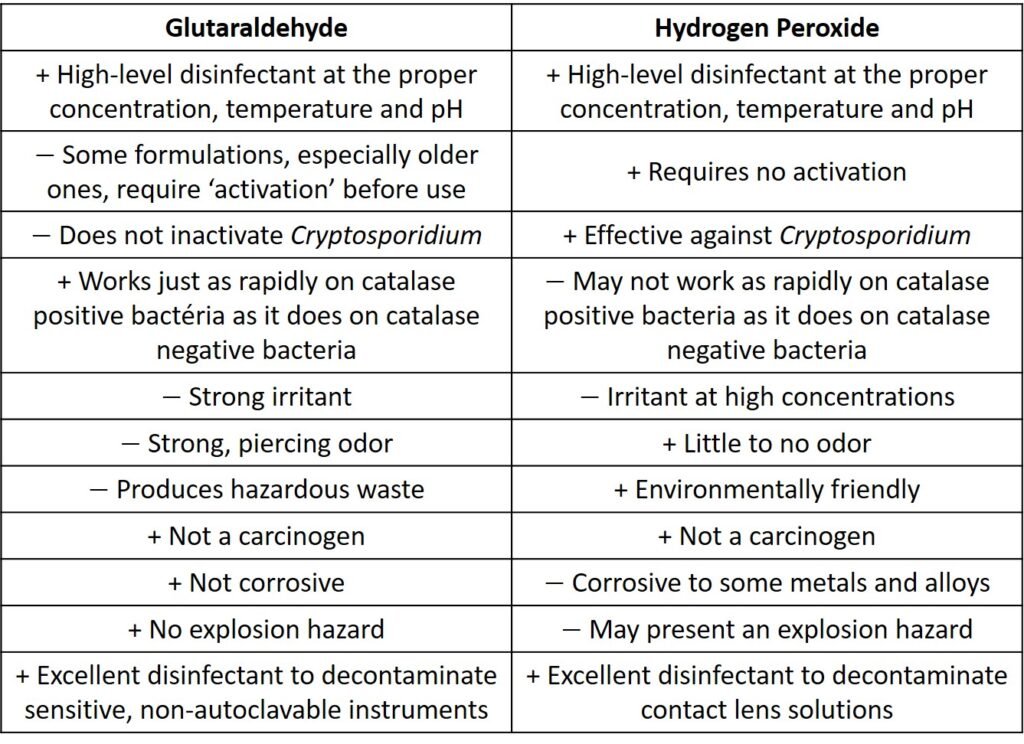
Some of the practical aspects of glutaraldehyde and hydrogen peroxide discussed are summarized in Table 2.
Table 2. Characteristics of gluraraldehyde and hydrogen peroxide
References
Download references list here.



