In this article, we investigate single-reaction gas-phase equilibria; specifically, we show how one can easily determine the equilibrium composition with knowledge of the equilibrium constant K. As the student surely knows, any typical chemical reaction can be represented by the general notation
where is a placeholder for the formula of a chemical species and
is a stoichiometric coefficient. The symbol
itself is a stoichiometric number, and by the sign convention adopted in most textbooks, it is associated with a positive sign (+) for a product or a negative sign (-) for a reactant. For instance, in the reaction studied in Example 1 below,
the stoichiometric numbers are
The number of moles of a given reactant/product is related to the stoichiometric number by an expression of the form
where we have introduced a variable , known as the reaction coordinate, which describes the extent or degree to which a reaction has taken place. Integrating the relation above gives
Summation over all species yields
The mole fractions of the species present are expressed as
In a gas-phase reaction, the molar fractions, the system presure, and the so-called equilibrium constant are related by an expression of the form
Here, is the molar fraction of component i,
is the fugacity coefficient of component i,
is the stoichiometric coefficient of species i,
is pressure,
is the reference pressure (usually taken as 1 bar),
is the reaction stoichiometric number, and
is the equilibrium constant. For ideal gases, the fugacity coefficient equals unity and the equation simplifies to
In words, the scary product on the left-hand side simply means that we should multiply in series the molar fractions of each component i, taken to a power equal to its stoichiometric coefficient. Consider, for instance, the simple equilibrium
Here, has a stoichiometric number
= -1 and
has
= 1. Denoting by
the molar fraction of dinitrogen tetroxide and by
the molar fraction of nitrogen dioxide, we write
The molar fractions can be shown to be
so that
Clearly, determining the extent of reaction becomes a matter of evaluating the equilibrium constant K. This quantity is expressed in terms of the reaction change in Gibbs free energy as
The value of at the reaction temperature T can be estimated with the relation
Here, subscript “0” denotes a quantity expressed at the reference temperature , usually 298 K. Given the heats of formation
and the change in Gibbs free energies of formation
, the values of
and
can be easily determined. The heat capacity integrals on the right-hand side can be computed with the general expressions
in which =
/
is the temperature ratio and the
‘s are, by definition,
Having evaluated all terms on the right-hand side of equation (III), the reaction change in Gibbs free energy and thence the equilibrium constant
can be established. Lastly, the extent of reaction and thence the molar fractions can be determined with equation (II). The steps are summarized below.
Step 1. With the initial number of moles and stoichiometric coefficients, determine expressions for the molar fractions using equation (I).
Step 2. Substitute the molar fractions into equation (II) to determine an expression that relates the extent of reaction to the equilibrium constant K.
Step 3. Determine the heat of reaction and the change in Gibbs free energy
at 298 K.
Step 4. Using the temperature ratio and the
coefficients, evaluate the heat capacity integrals.
Step 5. Using the results from steps 3 and 4, determine the right-hand side of equation (III).
Step 6. Knowing that , determine the equilibrium constant K.
Step 7. Substituting K in the expression determined in step 2, determine the extent of reaction .
Step 8. Substitute in the expressions derived in step 1 and determine the mole fractions.
We now present two applied examples.
Example 1
The following reaction reaches equilibrium at 500ºC and 2 bar:
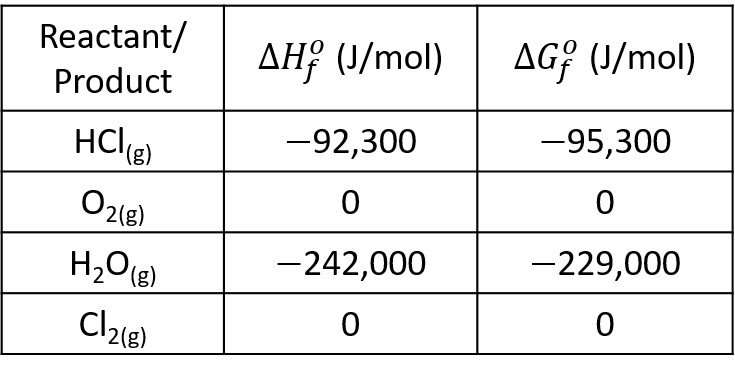
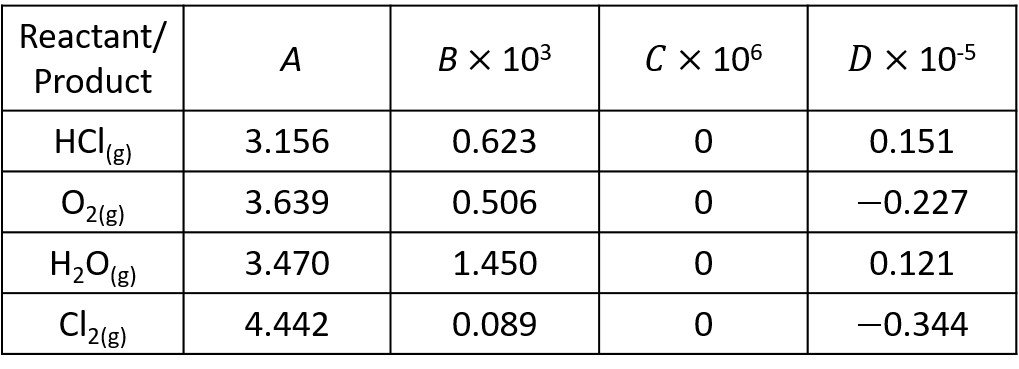
If the system initially contains 5 mol of HCl for each mole of oxygen, what is the composition of the system at equilibrium? Assume ideal gases. Use the following data.
The molar balance is outlined below.
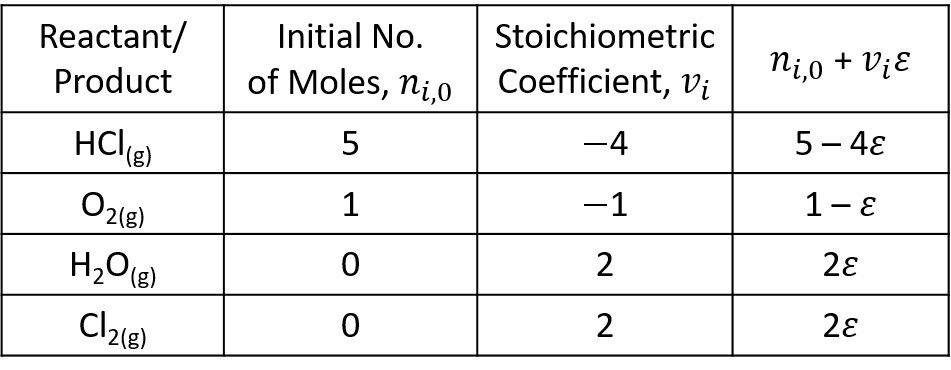
The total No. of moles at the beginning of the reaction is
The product of reaction stoichiometric number and extent of reaction is
The mole fractions of each component are written next.
The equilibrium constant, pressure, and composition are related by equation (II),
Substituting the molar fractions, = 2 bar,
= 1 bar, and
= -1 gives
It remains to compute the equilibrium constant K. In step 3, we calculate the heat of reaction at 298 K,
and the change in Gibbs free energy at 298 K,
Next, we compute the coefficients for use with the heat capacity integrals.
Now, the temperature ratio is = 773/298 = 2.59. In step 4, we evaluate the enthalpy integral
and the entropy integral
We now have all the information necessary to calculate . Indeed,
Resolving the logarithm,
Substituting in equation (IV) yields
Adjusting the fraction on the left-hand side, we have
The equation above is a fifth-degree polynomial in and does not lend itself to elementary methods of solution. One way to go is to apply Mathematica’s Solve command,
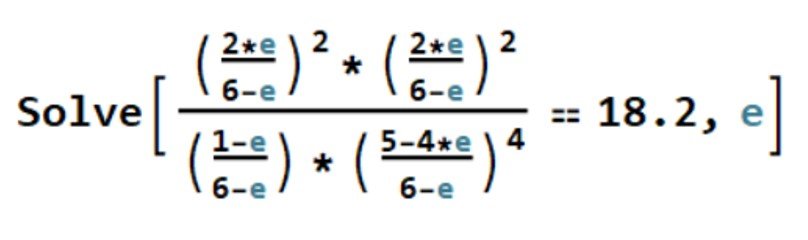
This returns four imaginary solutions and = 0.806, which is a valid result. The extent of reaction has been determined. It remains to compute the molar fractions, namely
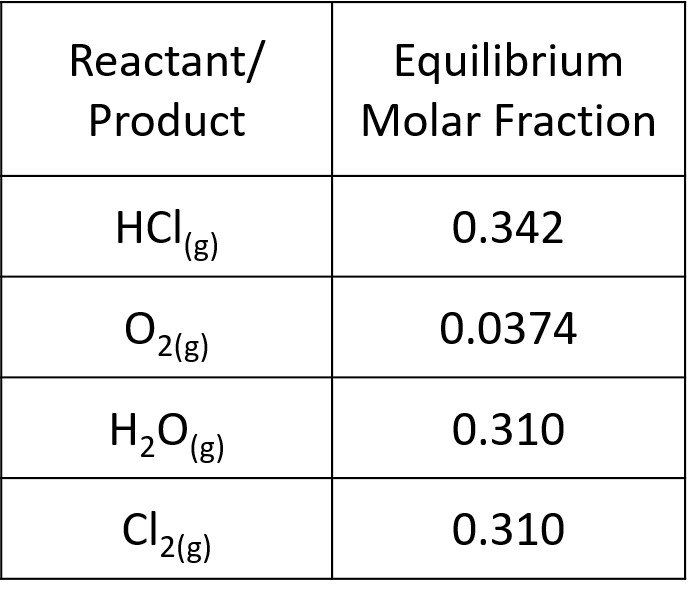
The sum of molar fractions is such that
as it should be. The slight difference is due to roundoff. Our results are tabulated below.
Example 2
Oil refineries frequently have both and
to dispose of. The following reaction suggests a means of getting rid of both at once:
For reactants in the stoichiometric proportion, estimate the molar fraction of each reactant if the reaction comes to equilibrium at 450ºC and 8 bar. Use the following data.
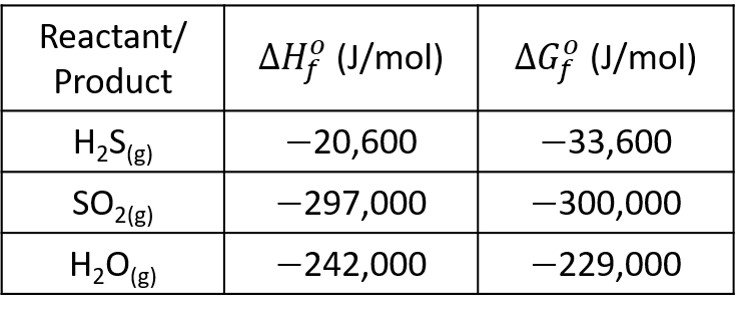
The sulfur exists pure as a solid phase, for which the activity is /
. Since
and
are for practical purposes the same, the activity is unit, and it is omitted from the equilibrium equation. Having established this, we turn to the molar balance of the three other species involved in the reaction.
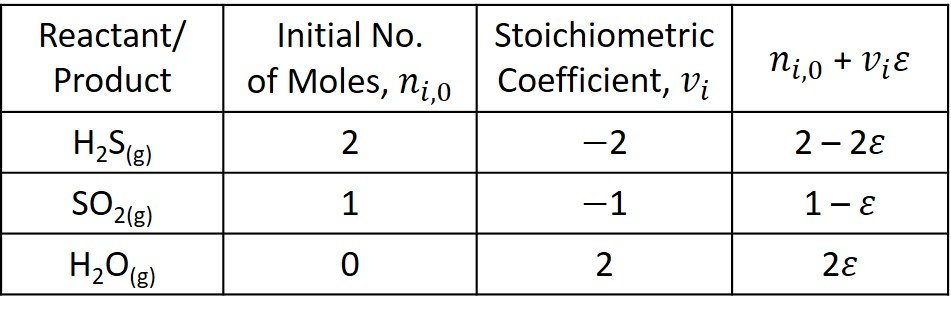
The total No. of moles at the beginning of the reaction is
The product of reaction stoichiometric number and extent of reaction is
The mole fractions of each component are written next.
The equilibrium constant, pressure, and composition are related by equation (II),
Substituting the molar fractions, = 8 bar,
= 1 bar, and
= -1 gives
The heat of reaction at 298 K is
and the change in Gibbs free energy at 298 K is
Next, we compute the coefficients for use with the heat capacity integrals.
Now, the temperature ratio is = 723/298 = 2.43. In step 4, we evaluate the enthalpy integral
and the entropy integral
We can now establish the value of ,
Resolving the logarithm,
Substituting in equation (V) yields
As before, we can solve the ensuing equation with Mathematica’s Solve command,
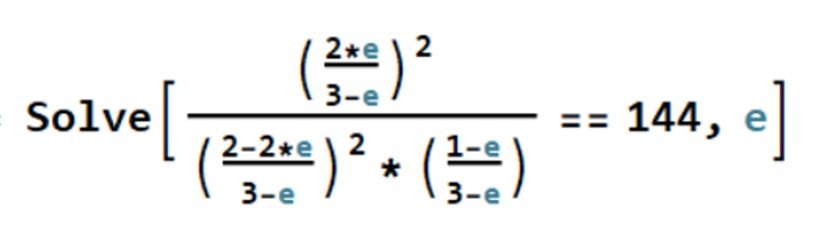
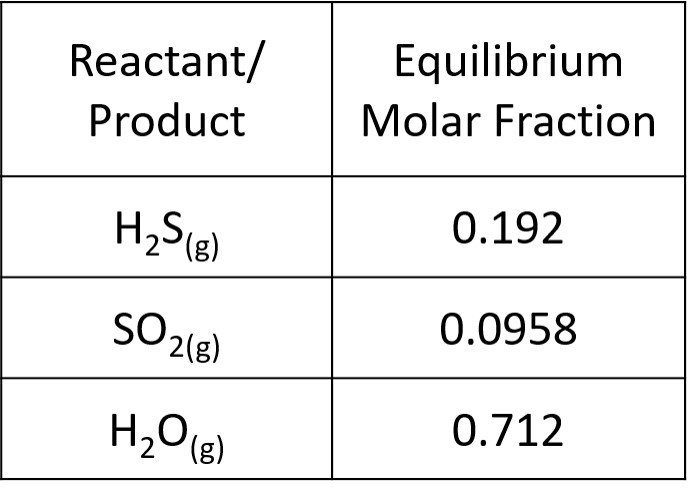
This returns two imaginary solutions and = 0.788, which is a feasible result. The extent of reaction has been established. Lastly, we can determine the equilibrium mole fractions,
The sum of molar fractions is
as one would expect. The results are summarized below.
Reference
• SMITH, J., VAN NESS, H. and ABBOTT, M. (2004). Introduction to Chemical Engineering Thermodynamics. 7th edition. New York: McGraw-Hill.




