1. Introduction
Cyanobacteria, the most numerous components of phytoplankton, are detested because of their potential to produce health-affecting toxins and odorous compounds that restrict the usage of lakes for practical applications, including supply of drinking water, irrigation, aquaculture, fish breeding, and recreation. When given the proper conditions, cyanobacteria can multiply to overwhelming number and volume, forming so-called harmful algal blooms (HABs).
There is substantial controversy on the factors that determine cyanobacterial dominance in freshwater systems. Since many bloom-forming cyanobacteria can fix atmospheric N2, some have reasoned that cyanobacteria should dominate at low N:P ratios. This hypothesis was endorsed by Smith (1983), who, relying on observations from 20 mostly northern European lakes, concluded that ambient N:P indeed influenced the dominance of cyanobacteria in the phytoplankton. However, subsequent analysis by Trimbee and Prepas (1987) pointed to several shortcomings of Smith’s methodology, including issues such as use of several years’ data from lakes undergoing significant changes in nutrient regime and treatment of data from multiple points within a lake as independent samples. Trimbee and Prepas (1987) went on to contend that, in the dataset that Smith had used, nutrient concentrations (e.g., total P or total N) were better predictors of average cyanobacteria dominance than N:P. Likewise, Downing et al. (2001) and Wagner and Adrian (2009) assessed several variables and found that N:P ratio may be a poor predictor of cyanobacterial abundance.
More recent research has shown that prediction of cyanobacterial dominance should not generalize the same nutrient gradient dependence to all blue-greens. For example, Dolman et al. (2012) found that nine cyanobacterial taxa differed substantially in response to changing total N and total P in the surrounding environment; interestingly, two Nostocales taxa, which are N2-fixing organisms, achieved greater biovolume in relatively high N low P enriched lakes, in contrast to what one might expect. There is also a growing body of research on how lacustrine cyanobacterial dominance may be affected by other factors, such as temperature (Kosten et al., 2012). Although cyanobacteria in, say, polymictic lakes may not profit directly from increased water temperatures, they benefit from longer stratification periods and an enhanced hypolimnetic nutrient supply, especially phosphorus (Wagner and Adrian, 2009). This indicates that lacustrine algal blooms may become more common and more intense with climate warming.
Indeed, there is growing evidence that cyanobacterial blooms are increasing globally; for example, an analysis of cyanobacterial pigments in sediment cores from over 100 lakes in North America and Europe has shown that cyanobacteria have increased substantially in 58% of the lakes since the industrial revolution, and this increase has accelerated since the mid-1940s (Taranu et al., 2015).
As eutrophication intensified globally in the second half of the 20th century, stakeholders responded by implementing different control and mitigation techniques. In this post, we briefly discuss five such techniques: use of chemicals, sediment dredging, hypolimnetic aeration/oxygenation, biomanipulation, and ultrasound. Some of these techniques (e.g., release of copper sulfate) are used to counteract algal blooms directly, while others (e.g., artificial oxygenation) are most often used post-eutrophication as a means to improve water quality.
2. Chemical treatment: Copper sulfate, herbicides, barley straw and hydrogen peroxide
2.1. Copper sulfate
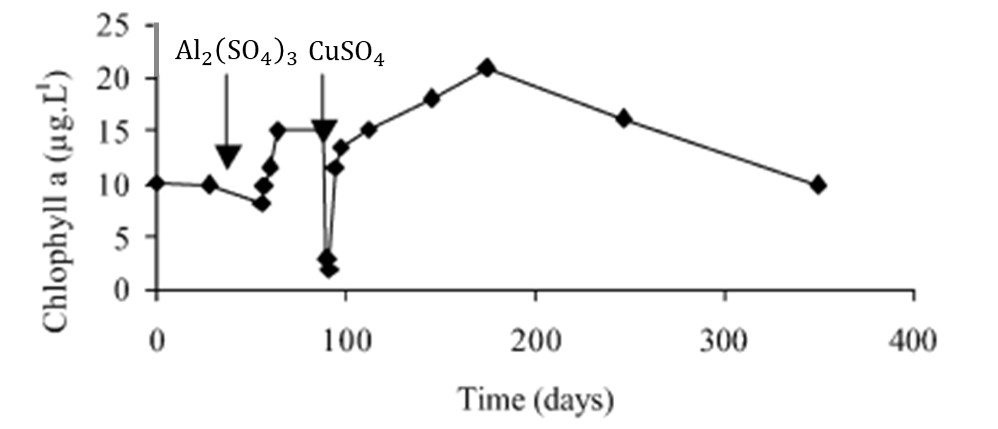
Used in lake management since at least 1904, copper sulfate was one of the first algicides. One of its main advantages is ease of application, as copper-based biocides occur in several presentations (most commonly in granular or crystalline form) and can be deployed in several ways. Although CuSO4 is the most common preparation, alternative sources of Cu include copper oxychloride, copper ethanolamine complex (cutrine), and copper citrate. Treatment levels reported in the literature range from 25 to 1000 g L-1 of Cu2+ depending on the physical chemistry of the water and the kind of application to the water body (McKnight et al., 1983). However, CuSO4 formulations generally have a limited effectiveness over time, as ionic copper is highly reactive in freshwater environments and can be rapidly converted into insoluble forms, diluted, or physically washed out of the system. This is exemplified in a detailed case study by van Hullebusch et al. (2002), who documented chemical treatment of Lake Courtille, a shallow polymictic lake in France. As shown in the chlorophyll-a time series in Figure 1, lake managers attempted to prevent a cyanobacterial bloom as summer approached by administering aluminum sulfate; this failed, as indicated by the rapidly increasing chla. Switching from prevention to mitigation, managers deployed copper sulfate and a precipitous decrease in chla was observed soon after. However, copper sulfate only temporarily inhibited cyanobacteria biomass growth and Microcystis strains were found again 2 months after the treatment.
Figure 1. Chlorophyll-a concentrations in Lake Courtille, France, as measured by van Hullebusch et al. (2002). The arrows indicate the times of introduction of aluminum sulfate and copper sulfate.
Further, copper sulfate is not at all biospecific, posing a threat to aquatic plants, aquatic insects, and zooplankton. The toxicity of copper to aquatic biota in standard laboratory conditions decreases in the order crustacean (10 g L-1); cyanobacteria (20
g L-1); algae and diatoms (20 – 100
g L-1); rotatoria, snails, amphibians, and submerged macrophytes (100 – 400
g L-1); and less sensitive organisms such as fish (400
g L-1 – 12 mg L-1) (Matthijs et al., 2016).
2.2. Herbicides
Herbicides were an obvious choice of algicide because they were thought to control cyanobacteria and green algae without affecting non-phototrophic life. Two herbicides commonly adopted in algae control practice are diuron and endothall. Diuron acts as an inhibitor of photosynthesis; while effective in removal of nuisance phytoplankton, it has a high persistence in sediments (up to 1 year) and can be environmentally degraded to 3,4-dichloroaniline, a genotoxic substance. Endothall acts by interfering on RNA synthesis; it is highly selective for cyanobacteria, but presents a serious toxicity hazard to some aquatic invertebrates. Development of resistance to endothall in algal populations, thereby calling for heavier dosages for the product to be effective, has also been reported (Prosecka et al., 2009).
2.3. Barley straw
In view of the potentially toxic nature of synthetic agents such as diuron and endothall, some stakeholders have advocated for use of natural products that exhibit algicidal properties. The most studied such product is barley straw, which has been considered for bloom control applications since the 1990s. Experiments conducted in the UK indicate that the application of barley straw at 25 – 50 g m–3 can suppress growth of various planktonic algae for 3 – 6 months, beginning 12 days to 2 months after application (Everall and Lees, 1997). Islami and Filizadeh (2011) exposed cultures of several species of cyanobacteria and green algae to barley straw extract; significant inhibition of growth was observed for a number of species, including eminently bloom-forming Microcystis aeruginosa and Anabaena flos-aquae. In a similar set of experiments with exposure to barley straw, Martin and Ridge (1999) indicated that four strains of M. aeruginosa were the most susceptible of the species they tested.
The chemical mechanism that underlies the algistatic activity of barley straw is unknown. Most researchers have been working with the hypothesis that the inhibitory activity arises from the oxidation of phenolic extracts released from the solubilized lignin found in aerobically decomposing barley straw. Waybright et al. (2009) contested the suggestion that the inhibitory polyphenol might be a tannin, because the straw extract they processed retained its activity after hydrolysis; tannins are known to be susceptible to hydrolysis. Iredale et al. (2012) reported the first experimental evidence that microbial activity is the primary process by which the active components of barley straw are released; Iredale’s team noted that peculiar fungal isolates have been found in early investigations of decomposing straw, and Murray et al. (2010) have shown that pre-treatment of straw with white rot fungi can reduce the lag period before straw becomes inhibitory to the green alga Scenedesmus.
Some algae appear to not be affected by barley straw unless the straw is applied in a way that keeps the water consistently oxygenated. This is an extension of laboratory observations such as those of Pillinger et al. (1994), who noted that rotted straw liquor only had a significant effect on the growth of Chlorella spp. if aeration was increased during the final 7 days of the decomposition period. According to Boylan and Morris (2003), two explanations for this dependence on oxygen might be that (1) oxygen must be supplied to the microbes that decompose the straw; or (2) the polyphenolic compounds associated with the algistatic activity are enhanced once they are oxidized. At any rate, Boylan and Morris (2003) showed that deployment of barley straw in stagnant ponds in Iowa, USA, failed to inhibit growth of Cladophora spp.
2.4. Hydrogen peroxide
Hydrogen peroxide, H2O2, has also been considered as a potential sustainable algicide product. HP is a potent oxidant, yielding highly reactive hydroxyl radicals that can damage biological cells and inhibit photosynthetic activity by disrupting photosystem II; it is highly selective, in that cyanobacteria are negatively affected at HP concentrations 10 times lower than those that may harm green algae and diatoms (Drábková, 2007); it is fast-acting, such that a lake treated by this chemical should be safe for swimming or other uses within no more than 3 days (Matthijs et al., 2016); it decomposes into water and hydrogen rather than potentially toxic molecules; lastly, it is affordable.
The higher sensitivity of cyanobacteria to H2O2 relatively to eukaryotic phytoplankton can be explained by their markedly different response to intense light stress (Weenink, 2015; Matthijs, 2016). Eukaryotic algae subjected to high light yield H2O2 as a byproduct of photosynthesis. Added light increases photosynthetic electron flow, causing the alga to reduce oxygen into superoxide anion (Mehler reaction). Superoxide, an example of Reactive Oxygen Species (ROS), is then enzymatically converted to H2O2 by superoxide dismutase. Lastly, H2O2 is converted to water and oxygen by catalase or, alternatively, anti-ROS enzymes such as ascorbate peroxidase reduce H2O2 to water. Cyanobacteria have no pathways to carry out a Mehler reaction. Rather, they respond to photochemical stress with a Mehler-like reaction that involves two flavodiiron proteins that produce water directly; no intermediary ROS compounds such as H2O2 are formed. It follows that oxidative stress levels are lower in cyanobacterial cells than in eukaryotic algae, thereby reducing the demand for high presence of ROS-eliminating enzymes such as ascorbate peroxidase, which is absent in most cyanobacteria. This makes cyanobacteria more sensitive to H2O2 than eukaryotic algae and higher plants.
As in the case of other algicides, however, the effectiveness of H2O2 in inhibiting algae is highly dependent on algae species and colony size. For example, Lusty and Gobler (2020) administered H2O2 to water samples from four eutrophic lakes and, upon assessing the ensuing microbial diversity, found that genera of cyanobacteria decreased in sensitivity to H2O2 in the order Planktothrix > Microcystis > Cylindrospermopsis. Lusty and his colleague argued that Cylindrospermopsis spp. may be particularly resistant to hydrogen peroxide because of its array of H2O2-degrading enzymes, including superoxide dismutase, catalase, and ascorbate peroxidase. Microcystis spp., in turn, may withstand H2O2 because they often form large globular colonies with cells surrounded by polysaccharide mucus, which contains extracellular polymeric substances with H2O2 scavenging abilities that may provide an antioxidant buffer to the cells within the colony. Liu et al. (2017) found that a treatment with H2O2 at 5 mg/L was insufficient to disrupt large Microcystis colonies, such that photosynthetic activity partially recovered after 48 h; a dosage of 20 mg/L, on the other hand, led to severe damage to Microcystis colonies, especially smaller ones, and fully disrupted metabolism regardless of colony size. While reliance on a higher dosage of H2O2 may ensure inhibition of the nuisance algae, it increases the risk of cyanobacterial cell lysis and subsequent release of intracellular toxins such as microcystins.
3. Sediment dredging
Internal phosphorus loading from enriched upper sediment layers can sustain a lake’s eutrophic condition long after external loading has been interrupted. Dredging of upper sediment layers may extinguish the water body’s phosphorus supply and thereby accelerate restoration efforts. Sediment dredging can be severe; working with Lake Okeechobee, Florida, USA, Reddy et al. (2007) calculated that removal of the top 30 cm sediment of that body of water would account for over 120 years of P loading.
One of the earliest cases of successful restoration via sediment dredging was in Lake Trummen, south-central Sweden (Björk et al., 2010). The lake had been used for swimming and water supply until the 1920s; however, deposition of sewage and other forms of pollution led to the lake’s hypertrophic collapse in the 1940s. Although wastewater discharge to the lake was interrupted between 1957 and 1958, the lake did not recover, with summer transparency still no greater than 20 cm. In the 1960s, local stakeholders launched a project to restore the lake by sediment dredging. The topmost half meter of sediment was removed by suction dredging in 1970, and another half meter was processed in 1971; about 400,000 cubic meters of sediment were removed in total. The benefits were immediate: cyanobacterial blooms disappeared, and a diverse plankton community replaced the monocultures of Microcystis that were dominant before restoration; the freshwater mussel Anodonta, which had been wiped out in the hypertrophic period, recolonized the lake; the phosphorus content of the water decreased from 600 g L-1 to 30
g L-1; the lake became once again suitable for sport fishing, swimming, and other forms of recreation.
Sebetich and Ferriero (1997) reported the use of sediment dredging in Kampfe Lake, New Jersey, USA, and compared its post-intervention characteristics with neighboring Glen Wild Lake, which was not dredged but had a similar developmental history and hence could be used as a control. Kampfe Lake exhibited superior conditions (Figure 2), including a mean TP throughout the water column of 20.9 g L-1, whereas Glen Wild maintained a mean TP of roughly twice this value. Kampfe Lake also had greater transparency and higher concentration of dissolved oxygen.
Laboratory experiments have indicated that, as in the case of other restoration techniques, the success of sediment dredging is contingent on control of external P loading (Kleeberg and Kohl, 1999; Zhong et al., 2007). This is especially true in the long term; Liu et al. (2016) assessed the effects of dredging in internal loading of N and P over 15 years in Lake Wuli, an urban lake in the city of Wuxi, China, and found that the release rate of soluble reactive phosphorus (SRP) had returned to pre-dredging levels 18 months after the intervention. They hypothesized that this was caused by the continued external pollution loading on the lake.
Li et al. (2020) studied the post-dredging dynamics in an aquacultural lake in China and found that this restoration technique is not a permanent panacea to eutrophication. Specifically, Li’s team found that post-intervention redeposition of natural sediment promotes the burial of bioavailable P and the transformation of inert P to active P, thereby increasing the risk of internal sediment P release and a return to eutrophication. In order to minimize the release of active P, they argued, dredging projects must incorporate other in situ management techniques such as transplanting submerged macrophytes, with the aim of increasing the depth of the oxide layer in surface sediments and avoiding sediment resuspension.
Figure 2. Concentration of total phosphorus contrasted between a dredged lake (Kampfe) and a similar non-dredged lake (Glen Wild) as measured by Sebetich and Ferriero (1997). The four sampling dates refer to the summer and fall of 1993.

4. Hypolimnetic aeration/oxygenation
Hypolimnetic aeration (HA) and oxygenation (HO) are techniques used to maintain or raise oxygen concentrations in the deepest layers of a lake through injection of air or oxygen. The phosphorus pool in the bottom sediment of a lake may be 100 times higher than the pool present in the lake water, implying a large dependence of P supply on sediment-water dynamics (Søndergaard et al., 2003). It has been shown that sediments release more phosphorus when they are anoxic. Hence, increased oxygen in the hypolimnion may reduce phosphorus release at the sediment-water interface and increase phosphorus retention, culminating in a reduction of trophic state. If algal bloom control is the objective, HA/HO reduces phosphorus concentrations in the hypolimnion to avoid entrainment of nutrient-rich bottom waters into the epilimnion (Bormans, 2016).
Mixed results have been achieved with hypolimnetic aeration and oxygenation. Schauser and Chorus (2007) described the restoration of Lake Tegel, Germany, through use of 15 aerators in conjunction with reduced external P loads. Using a modelling approach, they found that the aeration scheme would have produced no impact on the restoration effort if it were not accompanied by external load reduction. Liboriussen et al. (2009) reported that, of five dimictic Danish lakes that were oxygenated for 4 – 20 years, only minor improvements in oxic content of the hypolimnion were achieved, leading to summer concentrations no greater than 2.2 mg L-1; further, improvements were concentrated in the first months of stratification, and little improvement was obtained late in the season. Moore et al. (2012) documented the use of HO in Newman Lake, Washington state, USA, which, with a mean depth of 5.6 m, is one of the shallowest lakes in which oxygenation has been attempted. HO managed to prevent anoxia in most years of observation, but partial operation had to be replaced with full-capacity operation and eliminating oxygenation, even in midseason, was followed by a rapid depletion of hypolimnetic dissolved oxygen.
Of course, hypolimnetic aeration and oxygenation, albeit similar in principle, rely on different gases and may produce different effects. Austin et al. (2019) described use of partial-lift hypolimnetic aeration and linear-diffuser hypolimnetic oxygenation in two Minnesota lakes over the course of 30 years; oxygenation was better than aeration not only in terms of relief of hypoxia but also in oxidation of iron and manganese, suppression of internal P-loading, reduction of surface total phosphorus, and reduction of extremes of chla concentrations.
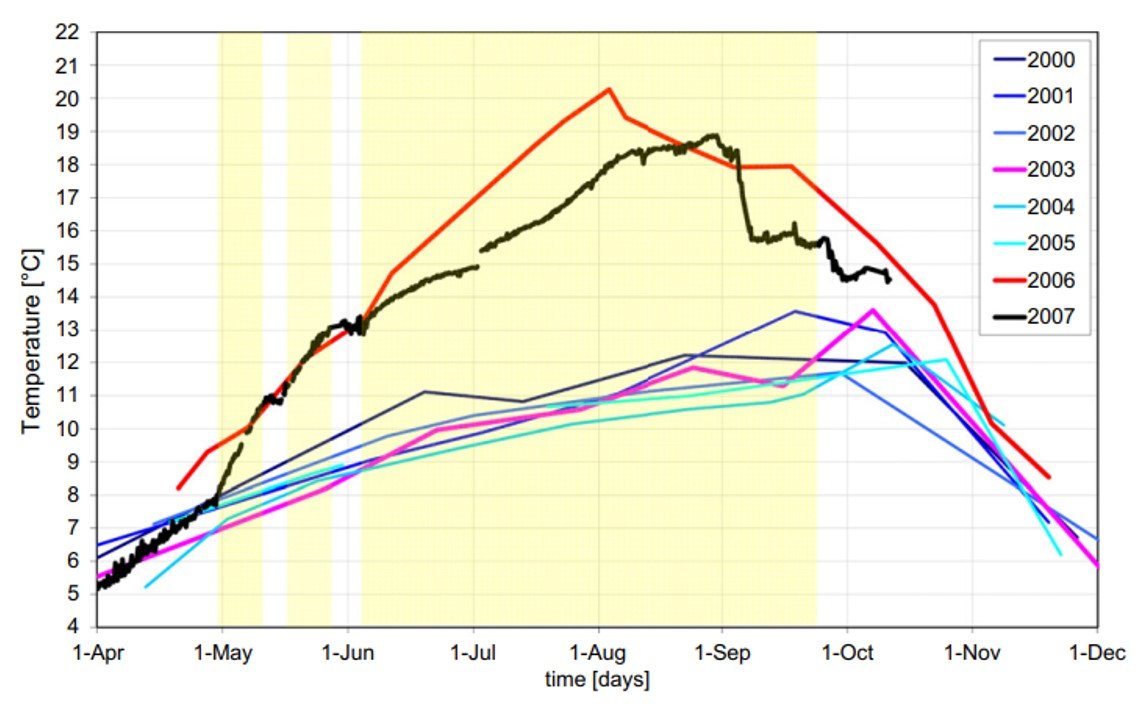
Hypolimnetic aeration and oxygenation are also associated with increased deep-water temperatures, which may weaken stratification and result in premature overturn in late summer. Working with data from HO in Serraia Lake, Italy, Toffolon et al. (2013) reported that deep-water temperatures achieved in summer rose by as much as 9oC after introduction of an oxygenation device (Figure 3). Toffolon’s team used a semiquantitative analysis to show that exothermic reactions associated with degradation of organic matter under newly established oxic conditions could not fully account for this increase. Rather, they argued, the greater temperature of the lake hypolimnion was a consequence of water mass circulation and augmented vertical diffusion induced by water injections at the bottom of the lake.
Figure 3. Seasonal behavior of the deep-water temperature in Lake Serraia, Italy, between 2000 and 2007 as reported by Toffolon et al. (2013). Oxygenation began in 2006; note the substantial increase in temperature for years 2006 and 2007.
5. Biomanipulation
High densities of plankti-benthivorous fish in lakes can prevent the growth of submerged macrophytes and drastically reduce the number and size of large zooplankton species, with the result that a phytoplankton-dominated state is achieved. A logical deduction, then, is that partial or complete removal of such fish may release zooplankton, increase grazing pressure, and promote reductions in harmful algae. This is an example of biomanipulation, that is, an ecosystem intervention based on deliberate modifications in an ecological community and its dynamics. Biomanipulation schemes of various types have been used as lake restoration techniques in the past 30 years. Due to the inherent difficulties of managing fish populations in large bodies of water, most biomanipulation efforts have been carried out in ponds and small lakes.
As mentioned above, the most common biomanipulation approach in freshwater is to remove zooplanktivorous and benthivorous fish. A reduction in the biomass of zooplanktivorous fish is generally followed by an array of ecological effects including reduced phytoplankton biomass, a shift to dominance by large zooplankton, improved transparency, and a higher proportion of piscivorous fish (e.g., perch and pike). Importantly, biomanipulation only yields the desired results if the extent of fish removal is great enough. A removal greater than 200 kg ha–1 over a three-year period has been suggested by Olin et al. (2006), who based this estimate on experience with Finnish lakes; Jeppesen and Sammalkorpi (2002) proposed that, in shallow temperate lakes, the annual amount of fish removal needed to produce significant improvements in water quality is given by the power law Catch-required = 6.9 TP0.52, where the catch required is in kg ha–1 and TP is total phosphorus in
g L–1.
A second approach to biomanipulation in freshwater environments is the stocking of piscivorous fish. The logic, in this case, is that piscivorous fish will prey on zooplanktivorous and benthivorous species, leading to a trophic cascade akin to the one that would have ensued if these species were simply removed. Commonly used piscivorous fish include walleye and largemouth bass in the USA and pike and pikeperch in the European temperate zone. Unfortunately, experience with biomanipulation via piscivorous fish has produced underwhelming results. For instance, Drenner and Hambright (2002) reviewed 17 studies on the subject and found that most did not indicate a piscivore effect on phytoplankton biomass. Some managers have used piscivore addition in conjunction with fish removal, but even in these cases some have contested the added value of the former technique relatively to sole use of the latter (Triest et al., 2016, and references therein).
Arguably, the greatest limitation of biomanipulation is its limited long-term effect, with several case studies noting a return to turbid waters and large populations of zooplanktivorous fish within 5 – 10 years. The most thorough report on long-term experience with biomanipulation is Søndergaard et al. (2008), who studied 36 Danish eutrophic lakes in which fish removal had been attempted for 10 – 15 years. In the first 6 – 8 years after fish removal, Søndergaard’s team noted a significant improvement in most variables of interest, including increased Secchi depth and lower chlorophyll-a, across most lakes. After approximately 10 years, however, most variables exhibited a tendency to return to pre-removal conditions. Reductions in the abundance of cyanobacteria were only statistically significant for the first 3 – 7 years.
Since biomanipulation interventions are often a one-time effort, more research on repeated fish removals is needed. One recent contribution, again from Denmark, is Søndergaard et al. (2017), who studied 30 years’ worth of data for restoration efforts in Lake Væng. The lake was subjected to fish removal twice, first in 1986 – 1988 and again in 2007 – 2009. Søndergaard’s team showed that the second biomanipulation replicated the chemical and biological effects of the first one even though 30% less fish was removed in the second operation. Thus, it appears likely that follow-up fish removals may be easier to implement and just as effective.
6. Ultrasound
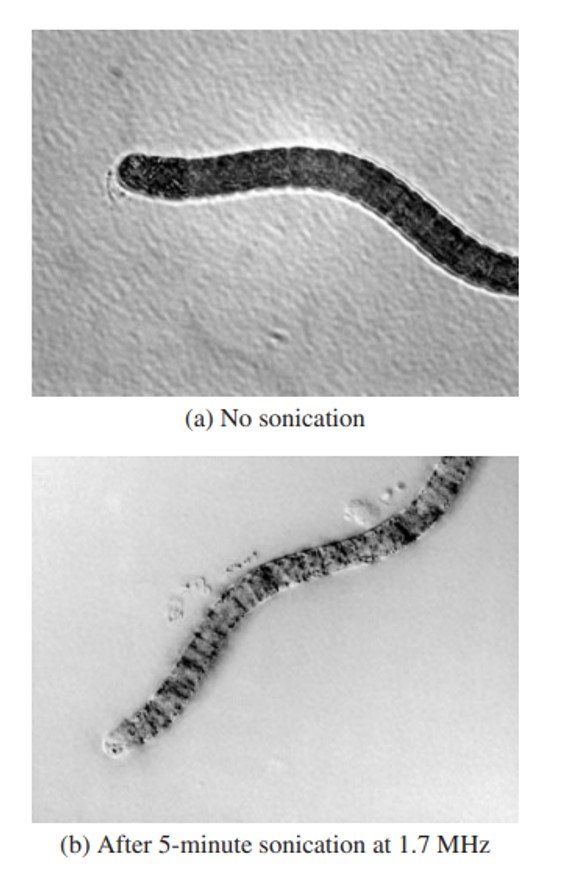
Ultrasonic irradiation was shown to be effective in controlling Spirulina and cyanobacterial species possessing gas vacuoles. Ultrasonic radiation in water causes a series of compression and rarefaction cycles that lead to the generation of millions of cavitation bubbles; implosion of these bubbles leads to temperatures as high as 5,000oC, pressures of the order of 500 atm, and formation of free radicals. Sonication inhibits the growth rate of cyanobacteria by rupture or collapse of gas vesicles owing to cavitational effects causing disruption of the cell wall and membrane, interruption of photosynthetic activity, and inhibition of cell division and the cell cycle (Figure 4). Zhang et al. (2006) noted that laboratory-scale removal of algae by sonication follows a first-order reaction.
Importantly, the extent of damage and thus control of algae by sonication is dependent on parameters such as frequency, intensity, and duration of exposure. Hao et al. (2004) tested ultrasonic sources at 20 kHz, 200 kHz, and 1.7 MHz to find that the inhibition effectiveness was greater for 200 kHz. Joyce et al. (2010) used 20, 40, 580, 864 kHz and 1.146 MHz at several intensities for 30 min and found that although 864 kHz gave maximum algal cell reduction, 580 kHz was the most efficient (with efficiency defined as percentage algal inactivation divided by intensity). Sonication at higher frequencies requires greater sound intensities, and therefore a greater electric power input, in order to achieve cavitation. Sonication at higher power intensity has been associated with more thorough removal of algae, but is often accompanied by cell lysis and accumulation of extracellular toxins (Zhang et al., 2006; Park et al., 2019). Likewise, greater exposure times lead to greater exposure to cavitation effects and, eventually, cell lysis. That being said, ultrasound is known to be effective for removal of algal toxins, and some workers have reported that prolonged sonication of algae such as M. aeruginosa, at least in laboratory settings, may in fact lower harmful substance levels (Rajasekhar et al., 2012). Whether extended deployment of ultrasound in the field leads to accumulation or depletion of algal toxins is still a topic of active research, although current evidence favors the former.
Figure 4. Differential interference microscopy of Arthrospira platensis (a) intact; and (b) after 5-minute sonication at 1.7 MHz. The damage to the alga cell is clear.
References
Download reference list here.




