Biomass is considered a promising source of renewable energy for future applications. Unlike fossil fuels, biomass is readily available anywhere in the world; it is easily stored and transported; and it can be processed in various ways to yield a variety of products ranging from bio-oils for lubrication to gaseous hydrogen for fuel cells. In this article, we focus on gasification, a biomass processing technique that has shown great potential for large-scale deployment.
1. Gasification
Gasification is a thermochemical process in which a solid fuel like biomass or coal undergoes sub-stoichiometric oxidation and reduction reactions in a gasifier reactor, yielding a mixture of CO, H2, CH4, and CO2. Additional products include condensable hydrocarbons collectively named ‘tar’ and particulate matter consisting of dust, soot, char, and ash. Other trace gases like hydrogen sulphide (H2S), hydrochloric acid (HCl), hydrogen cyanide (HCN), and ammonia (NH3) are also generated, albeit at very low concentrations.
When air or oxygen is employed as the gasifying medium, biomass gasification may be regarded as a kind of partial combustion. One important difference between biomass gasification and combustion, however, has to do with purpose: the former is primarily carried out to yield valuable gaseous products whereas the latter is mainly intended to generate heat. Furthermore, gasification is considered to be more environmentally sound than combustion because of its lower output of atmospheric pollutants and the wide array of applications of gasification products (Zhang et al., 2010).
One of the most prized products of biomass processing is hydrogen gas, which is a high-heating-value, environmentally innocuous diatomic fuel. At present, most industrial H2 is extracted from steam reforming of natural gas, which is not at all a sustainable process. Gasification of biomass produces a syngas rich in hydrogen if steam or oxygen and steam are used as the gasification agent. If air is used as a gasification medium, the atmospheric nitrogen dilutes the syngas, leading to a low hydrogen concentration and a high effort of hydrogen purification.
Syngas from biomass gasification can be further processed by the Fischer-Tropsch process to yield methanol, dimethyl ether, and other chemical feedstocks.
2. Biomass
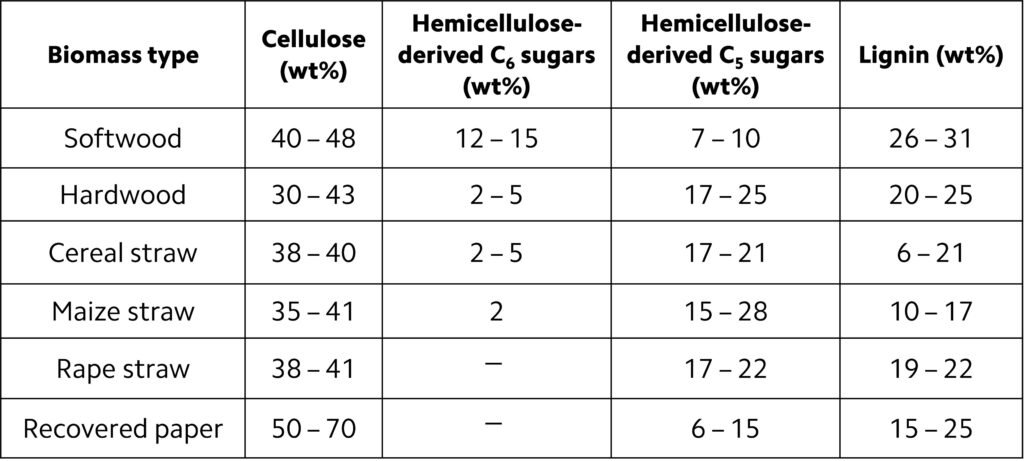
The most prominent constituent of biomass is lignocellulose, which consists of the non-starch, fibrous part of plant material. Cellulose, hemicellulose and lignin are the three main elements of biomass. Unlike, say, carbohydrates or starch, lignocellulose is not easily digestible by humans, hence its application as a fuel does not affect the world’s food supply. In a typical biomass, the cellulose-to-lignin ratio varies from 0.5 to 2.7 and hemicellulose-to-lignin ratio ranges from 0.5 to 2.0. The proportions of cellulose and hemicellulose are directly related to the yield of gaseous products, while the lignin content determines the oil in the product. Accordingly, the higher the ratio of cellulose and hemicellulose to lignin in a given biomass, the higher the gaseous product yields obtainable from gasification. Table 1 lists the chemical composition of some biomass products.
Table 1. Chemical composition of common lignocellulose feedstock.
The characteristics of the exit gases and optimal operation of the gasifier are also affected by moisture content. Most gasifiers are designed to process biomass feedstock with moisture content in the range 15 – 30 wt%. The problem with higher moisture contents is not exactly the inability of reactors to process the material, but rather the energy penalty incurred by the drying treatment that precedes gasification. For every kilogram of moisture in biomass, at least 2260 kJ of extra energy is needed to evaporate the water (Sikarwar et al., 2016; Mishra and Upadhyay, 2021).
3. Types of gasifier
Three main reactor designs are used for biomass gasification: fixed bed gasifiers, fluidized bed gasifiers, and entrained flow gasifiers.
Fixed bed reactor: updraft and downdraft (Figs. 1a and 1b)
A fixed bed gasifier can be either updraft (fuel enters from the top and the gasifying agent from the bottom) or downdraft (both fuel and gasifier enter from the top).
In a typical updraft gasifier, fuel is fed from the top; the product gas leaves from the top as well. The gasifying agent is preheated and fed into the gasifier through a grid at the bottom. The gas then rises through a bed of descending fuel or ash in the gasifier chamber. The gasifying medium, as it enters the bottom of the bed, meets hot ash and unconverted chars descending from the top. The temperature in the bottom layer well exceeds the ignition temperature of carbon, so a highly exothermic combustion reaction takes place in the presence of excess oxygen; the reactor temperature rises to 1000oC. The released heat heats the upward-moving gas as well as the descending solids.
The reaction above is fast and rapidly consumes the oxygen content in the reactor. In an atmosphere with rarefied oxygen, incomplete combustion follows, generating CO and some additional heat:
The hot gases, now largely composed of a mixture of CO, CO2, and steam, percolate upwards through the bed. The carbon dioxide in the rising gas increases rapidly in the combustion zone; once the oxygen is mostly depleted, CO2 enters the reaction with char:
Likewise, steam reacts with char:
The two foregoing reactions drive the formation of H2 and CO in the final product; since these reactions are endothermic, they are accompanied by cooling of the reactor, usually to 750oC.
The zone above the gasification zone is for the pyrolysis of biomass. The heat afforded by the rising hot gas heats up the dry biomass descending from above. The biomass is then decomposed (pyrolyzed) into a mixture of noncondensable gases, condensable gases, and char. Importantly, the product gas of updraft reactors has a high tar content because the tar, newly formed during pyrolysis, is not given the opportunity to pass through the combustion zone. Updraft gasifiers typically produce between 10 and 20 wt% tar in the produced gas, which is exceedingly high for many applications (Zhang et al., 2010).
The downdraft gasifier is another fixed bed gasification system. In contrast to the updraft gasifier, the air in a downdraft reactor is introduced into the reactor from a lower section, usually in a so-called ‘throated’ (i.e., narrow) region. In this region, the fuel feedstock injected from above is either burned or pyrolyzed. The combustion gases then pass down over the hot char at the bottom of the bed, where they are reduced to H2 and CO. The high temperature within the throat ensures that the tars formed during pyrolysis are significantly cracked (homogeneous cracking), with further cracking occurring as the gas meets the hot char on the way out of the bed (heterogeneous cracking). The final result is a less tarry gas, although simply replacing an updraft fixed bed system with a downdraft one by no means guarantees that the gasified product will be compatible with the most stringent biofuel applications.
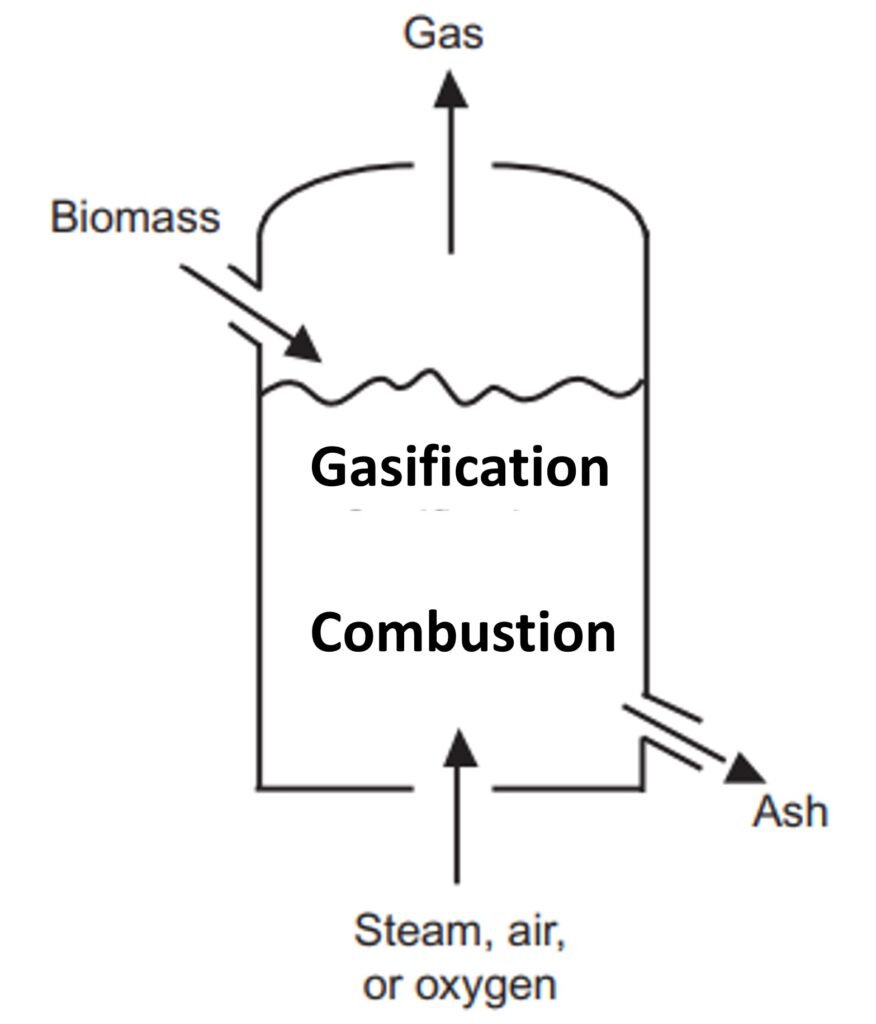
Fluidized bed reactor (Fig. 2)
Unlike other reactor types, a fluidized bed gasifier contains a bed of inert materials that serves as heat carrier and mixer, while the gasifying medium acts as the fluidizing gas. Biomass particles are heated to bed temperature (as a result of contact with hot bed solids) and undergo rapid drying and pyrolysis, producing char and gases. Subsequent gasification reactions take place further up as the gas rises. The bubbles of the fluidized bed can act as the primary conduit to the top, but, while they help in mixing, the bubbles can also allow gas to bypass the solids without participating in the gasification reactions (Basu, 2013). The pyrolysis products coming in contact with the hot solids break down into noncondensable gases, and, if they escape the bed and rise into the cooler freeboard, tar and char are formed.
Some of the shortcomings of bubbling fluidized bed reactors can be avoided by use of a circulating fluidized bed (CFB) reactor. In this reactor architecture, the hot bed material is circulated between the reactor and a cyclone separator, promoting intense mixing and longer solid residence times. The absence of any bubbles avoids the gas-bypassing problem of bubbling fluidized beds.
Fluidized bed technology is considered the most promising approach to biomass gasification because of its potential to gasify a wide range of fuels, a high mixing capacity, high heat/mass transfer rates, and a reliable compatibility with catalysts used to enhance tar reforming.
Entrained flow reactor
In an entrained flow gasifier, the feed is powdered to small sizes (< 75 m) and injected into a chamber along with oxygen and steam. The mixture is then subjected to high pressures, varying between ~20 and ~70 atm, and very high temperatures, often greater than 1300oC. This combination of severe conditions allows for rapid feed conversion and a high throughput of syngas (Zhang et al., 2010). The high operating temperature results in complete destruction of tar, hence entrained flow gasifiers are advantageous when more thorough tar removal is demanded. To facilitate feeding into the reactor, the fuel may be mixed with water to prepare a slurry, which may call for higher oxygen consumption and a greater reactor volume for evaporation of large amounts of water.
Figure 1. Fixed-bed gasifiers: (a) updraft and (b) downdraft.
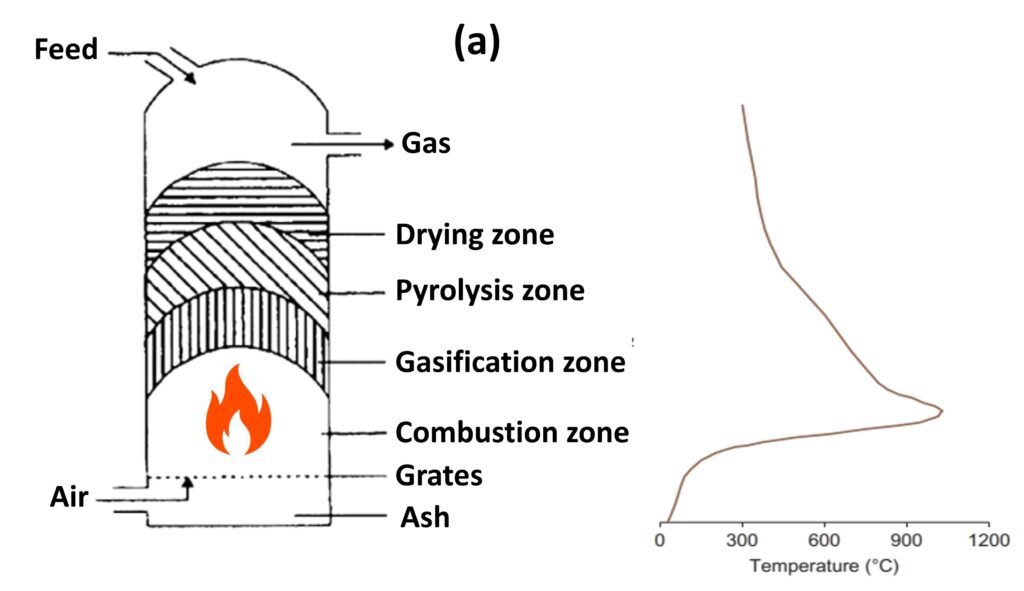

Figure 2. Fluidized bed gasifier.
4. Supercritical water biomass gasification (SCWG)
As the name implies, SCWG utilizes water in the supercritical state, wherein it exhibits remarkable thermomechanical properties that can be harnessed to dilute and process organic chemicals that are insoluble in ‘normal’ water. The gaseous output of SCWG is similar to other biomass gasification techniques, but with lower CO. Temperature seems to be particularly important: at temperatures below 450oC, CH4 is the main component of the gas product, whereas at reaction temperatures above 600oC hydrogen is dominant (Heidenreich and Foscolo, 2014). These trends are illustrated in Figure 3, which is a plot of the gas yield of SCWG as a function of temperature.
One outstanding advantage of SCWG is its ability to process wet biomass with no need for pre-drying; even liquid biomass such as olive mill water can be utilized for production of H2 gas via SCWG (Farzad et al., 2016 and references therein). While most experimental work on SCWG has involved lignocellulosic biomass, its compatibility with other forms of feedstock, such as sewage sludge, is well documented (Correa and Kruse, 2017). Hydrogen yields as high as 76 vol% have been reported by Onwudili et al. (2013) when gasifying sewage sludge at 450oC and 34 MPa for 1 h in a batch reactor with 3 g of NaOH as catalyst.
Predictably, the main limitation of SCWG is the large energy input needed to heat and pressurize water to the supercritical state. Researchers have tried to reduce reaction temperatures and enhance H2 yields by introducing catalysts such as Ni, Ru, activated carbon, and alkali metal-based materials including trona, KOH, and NaOH. Still, the cost of hydrogen production from SCWG continues to be appreciably higher than hydrogen obtained from steam reforming of methane, and the technology still has ways to go before it can be considered competitive.
Figure 3. Gas yield composition of supercritical water biomass gasification at different temperatures.

5. The tar problem
Tar is a thick, black, highly viscous liquid that can condense at low-temperature zones of a gasifier or end-use devices. Tars may polymerize to more complex structures in heat exchangers, exit pipes, or on filters for particulates, which could lead to attrition, choking, and other undesirable phenomena. Moreover, tar may retain over 10% of the higher heating value of gasification products.
The allowable tar levels in syngas depend on the application. As a rule of thumb, tar concentrations of 0.05 g Nm‒3, 0.005 g Nm‒3, and 0.001 g Nm‒3 are considered adequate for gas engines, gas turbines, and fuel cells, respectively (Sikarwar et al., 2016).
There is no universally accepted definition for tar, but some tentative ones have been proposed:
- The mixture of chemical compounds which condense on metal surfaces at room temperature;
- The sum of components with boiling points higher than 150oC;
- All organic contaminants with a molecular weight larger than benzene.
In a 1998 joint meeting of the International Energy Agency (IEA) Bioenergy Gasification Task, the Directorate General for Energy of the European Commission, and the US Department of Energy, tars were formally defined as the hydrocarbons with molecular weight higher than benzene (Maniatis and Beenackers, 2000).
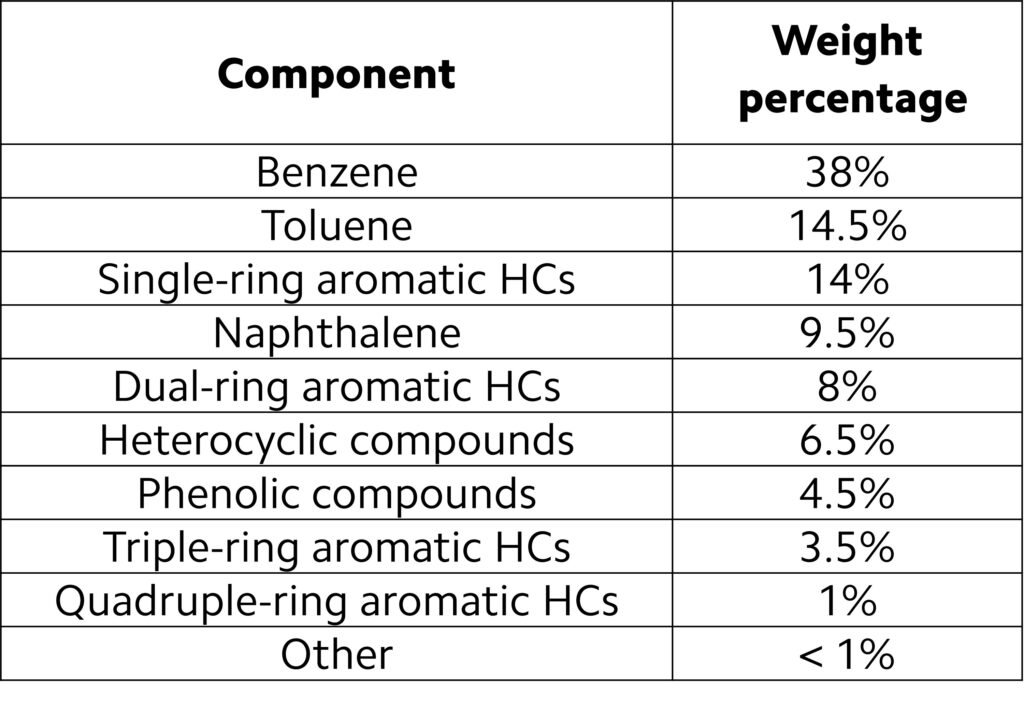
An approximate composition of tar is outlined in Table 2. The composition listed on this table is oversimplified, as tar may involve thousands of organic compounds and inorganic impurities such as H2S, HCl, and alkali metals, all of which are not listed on the table. In addition, the composition of tar is sensibly dependent upon the starting biomass; for instance, Jordan and Akay (2012) reported that quadruple-ring aromatics may account for more than 6.5 wt% (dry basis) of tars from gasified cane bagasse.
Table 2. Typical composition of tar. (HC = hydrocarbon.)
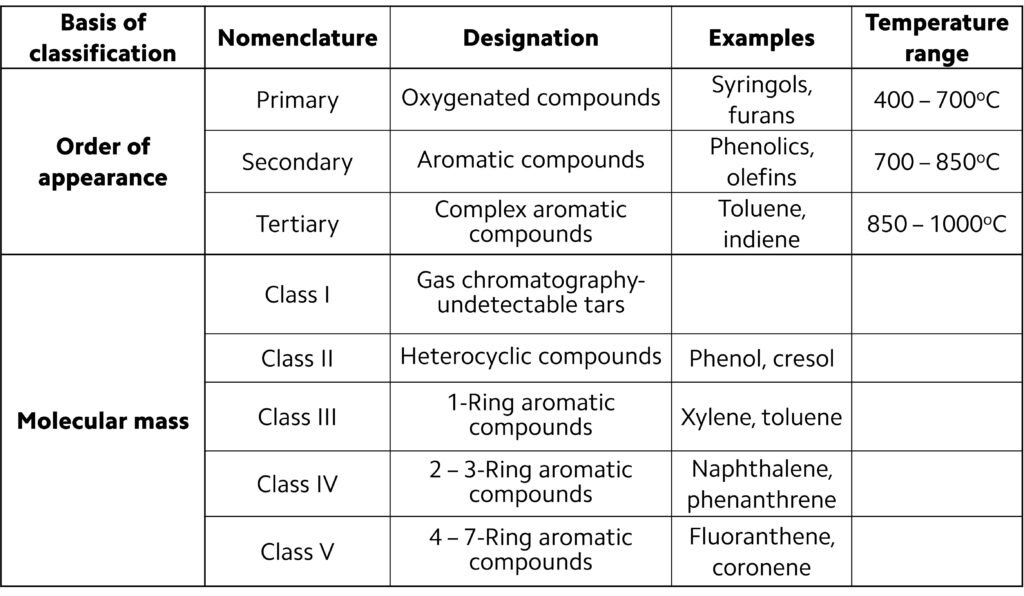
One widely used classification scheme for tars is based on the evolution of biomass oxygenates (vapors) obtained as the temperature is gradually increased from ~400oC to ~1000oC; this is illustrated in Figure 4. Tars can also be classified on the basis of molecular mass; typically, five categories or ‘classes’ are adopted, as summarized in Table 3.
Figure 4. Tars obtained from processing of lignin biomass.
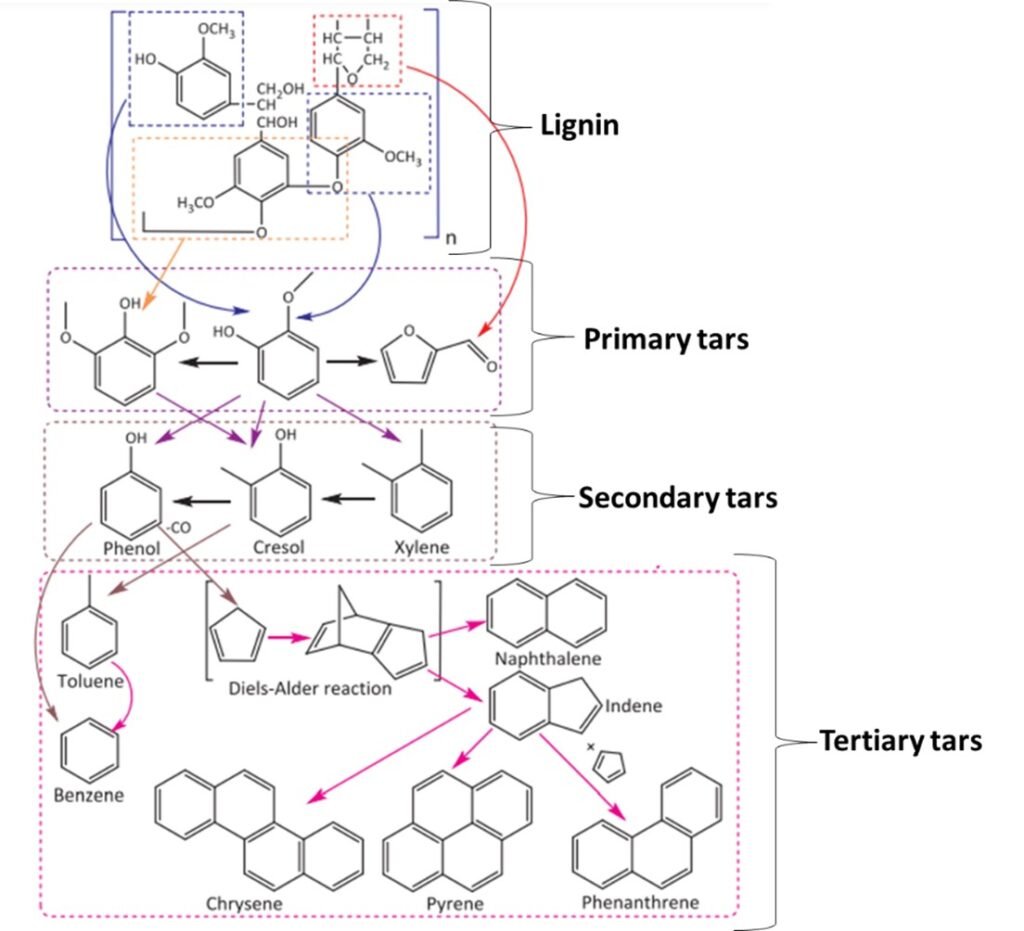
Table 3. Tar classification schemes.
The most straightforward chemical method for tar removal is thermal cracking, whereby high temperatures, usually in the range 1100 – 1300oC, are imposed to decompose large organic compounds. Brandt and Henriksen (2000) showed that at a temperature of 1250oC an exposure of no more than 0.5 sec was needed for effective reduction of tar. Research has also been carried out on tar removal via combination of thermal treatment with other process conditions. For example, Phuphuakrat et al. (2010) studied the effect of temperature and equivalence ratio (i.e., the ratio of the amount of air supplied to the stoichiometric amount of air necessary for complete combustion of the feedstock) on the tar yield of a small-scale sewage sludge gasifier; they reported that, with temperature maintained at approximately 1000oC, an increase in equivalence ratio from 0.30 to 0.32 sufficed to reduce syngas tar content from 11.8 to 6.56 g/m3.
Catalytic steam reforming is an attractive method in removal of tar while simultaneously enhancing syngas production. With the use of catalysts, steam and dry reforming reactions can be carried out at lower temperatures. Of the many catalyst types used in steam reforming, metal-based ones seem to be particularly effective in the removal of tar. In turn, nickel-based catalysts are preferred due to features such as high activity, low cost, easy regeneration, and, perhaps most importantly, previous experience with these catalysts in methane and naphtha reforming in the petrochemical industry (Saleem et al., 2020). Zhang et al. (2004) investigated catalytic destruction of tar over commercial Ni-based catalysts, aiming to improve the yield of syngas. Three specific commercial Ni-based catalysts were shown to be effective in removing heavy tars with destruction efficiencies of over 99%. What’s more, hydrogen yield was increased by 6 – 11 vol% (dry basis) in the presence of the Ni catalysts.
Numerous other catalysts have been investigated for tar removal, including dolomite, olivine, zeolite, and alumina. Particular research effort has been devoted to dolomite, which is inexpensive and readily available. Roche et al. (2014) studied the gravimetric tar composition in sewage sludge gasification catalyzed by dolomite; tar removal efficiency in the presence of dolomite was 36 – 48% when the gasifying agent was air, and 63 – 71% when the gasifying agent was air + steam. Andrés et al. (2011), comparing the catalytic performance of dolomite, olivine and alumina, noted that dolomite was not only the most effective catalyst for tar reduction, it also increased the lower heating value of syngas. The main limitation of dolomite catalysts is their poor attrition resistance, which in operational terms may lead to the formation of unwanted particulates in the reactor product stream so that frequent catalyst replacement is made necessary (Yung et al., 2009).
As summarized by Islam (2020), there is also a substantial body of research on the use of dolomite in conjunction with other catalysts, such as activated carbon and nickel. For example, Wang et al. (2005), working with naphthalene as a representative tar compound, noted that use of a Ni/dolomite catalyst led to tar reductions of nearly 95% within one hour of reactor operation; the hydrogen yield also increased.
References
Download references list here.